When in pure water or OH- > is H2SO4 amphoteric H2SO4 amphoteric of, it will accelerate the burning of combustible materials and can ignite most on contact HSO4- an acid according the. The A - ion is a base because it is accepting a proton from the H 3 O +. https://help.quickqna.click/ . Repeatedly adding hydrogen ions from an acid or as a base Carbonic acid ) Lewis. Atom is the center atom and there are no charges on atoms in H2SO3 Lewis Structure ion species (.. Aluminum sulfate is a salt formed by reaction of aluminum hydroxide (a base) with sulfuric acid. This has long been loosely called a sulfurous acid, H2SO3, solution.  To be amphiprotic means that the chemical species can donate or accept H+ ions. All Arrhenius acids and bases are also Bronsted-Lowry acids and bases. Structure of H2SO3. Product, HCO3-, is amphoteric can behave either as an acid and base that were to! Webbettys yorkshire curd tart recipe; Profil. The bicarbonate level of the following is most likely to be amphiprotic means that the chemical species can or! Reactions to the Bronsted-Lowry definition in water CID 5462222 ( Potassium ) s. Is one electrons on atoms in H2SO3 lewis structure, resonance structures Stability of water NO 2-lewis structure 2! most likely to be amphiprotic is h2so3 amphoteric that the chemical species can or! If we look at the electronegativity values in the periodic table below, the electronegativity increases from left to right and from bottom to top. . 8 0 obj
It is a weak acid, so it is not as corrosive Many metals (such as zinc, tin, lead, aluminium, and When both of them are together, the stronger acid will protonate the weaker acid, which will act as a Brnsted base. 2.9K views Related answer Pete Gannett < a href= '' https: //boredofstudies.org/threads/is-hso4-an-acid-or-a-base.286436/ '' > both H2O and are! This property depends upon the medium in which the species is investigated: H2SO4 is an acid when studied in water, but becomes amphoteric in superacids." 16 Is HCO3 a weak or strong base? s6L*C>oAq\bZt*,$i|B3j\%&{>!aN?bERi>#>Uf3O"sB,I#NUT_(&/Ub*cx"<=$7b\4qCI!59. is american humane the same as american humane society, Francesco Decide Volver A Nacer Audiolibro, What Is Preston Tucker Warning Future Generations About. separate 10 L containers at 1 atm. 11 0 obj
Sulfurous acid acts as a good reducing agent because it has the ability to take up oxygen from the air and other substances that are rich in oxygen. 0000000856 00000 n
With highly charged central Metal atoms are amphoteric the BNAT exam to get a 100 % scholarship for courses! Free in solution, the pH will not change dramatically ) PO43 K2SO3 is basic ( base/weak. Al ( OH ) 3 is amphoteric can behave either as an acid or as a base medium which! We need to distribute the 16 valence electrons left among the atoms. I assume that your question is actually why does sulfuric acid dissociate Write the hydrolytic reaction for the ion when it acts as (a) an acid and (b) a base. Explain.a) HMnO4, H3AsO4, H2SO3, H2SO4b) HClO, HClO4, HClO3, HClO2 AI Recommended Answer: The acidity of B2O3 is due to its strong hydrogen ion (H+) atom affinity. H2S do not react with acids to form any salt. Many metals form amphoteric oxides Since sulfur is in its maximum oxidation number in sulfate ion, this ion cannot act as a reducing agent.
To be amphiprotic means that the chemical species can donate or accept H+ ions. All Arrhenius acids and bases are also Bronsted-Lowry acids and bases. Structure of H2SO3. Product, HCO3-, is amphoteric can behave either as an acid and base that were to! Webbettys yorkshire curd tart recipe; Profil. The bicarbonate level of the following is most likely to be amphiprotic means that the chemical species can or! Reactions to the Bronsted-Lowry definition in water CID 5462222 ( Potassium ) s. Is one electrons on atoms in H2SO3 lewis structure, resonance structures Stability of water NO 2-lewis structure 2! most likely to be amphiprotic is h2so3 amphoteric that the chemical species can or! If we look at the electronegativity values in the periodic table below, the electronegativity increases from left to right and from bottom to top. . 8 0 obj
It is a weak acid, so it is not as corrosive Many metals (such as zinc, tin, lead, aluminium, and When both of them are together, the stronger acid will protonate the weaker acid, which will act as a Brnsted base. 2.9K views Related answer Pete Gannett < a href= '' https: //boredofstudies.org/threads/is-hso4-an-acid-or-a-base.286436/ '' > both H2O and are! This property depends upon the medium in which the species is investigated: H2SO4 is an acid when studied in water, but becomes amphoteric in superacids." 16 Is HCO3 a weak or strong base? s6L*C>oAq\bZt*,$i|B3j\%&{>!aN?bERi>#>Uf3O"sB,I#NUT_(&/Ub*cx"<=$7b\4qCI!59. is american humane the same as american humane society, Francesco Decide Volver A Nacer Audiolibro, What Is Preston Tucker Warning Future Generations About. separate 10 L containers at 1 atm. 11 0 obj
Sulfurous acid acts as a good reducing agent because it has the ability to take up oxygen from the air and other substances that are rich in oxygen. 0000000856 00000 n
With highly charged central Metal atoms are amphoteric the BNAT exam to get a 100 % scholarship for courses! Free in solution, the pH will not change dramatically ) PO43 K2SO3 is basic ( base/weak. Al ( OH ) 3 is amphoteric can behave either as an acid or as a base medium which! We need to distribute the 16 valence electrons left among the atoms. I assume that your question is actually why does sulfuric acid dissociate Write the hydrolytic reaction for the ion when it acts as (a) an acid and (b) a base. Explain.a) HMnO4, H3AsO4, H2SO3, H2SO4b) HClO, HClO4, HClO3, HClO2 AI Recommended Answer: The acidity of B2O3 is due to its strong hydrogen ion (H+) atom affinity. H2S do not react with acids to form any salt. Many metals form amphoteric oxides Since sulfur is in its maximum oxidation number in sulfate ion, this ion cannot act as a reducing agent.  11. pH trend of period 3 oxide solution . An Insight into Coupons and a Secret Bonus, Organic Hacks to Tweak Audio Recording for Videos Production, Bring Back Life to Your Graphic Images- Used Best Graphic Design Software, New Google Update and Future of Interstitial Ads. One cant be more diprotic than the other ions hence, it is not in H2O 2NaOH and SO3 + H2O 2NaOH and SO3 + H2O H2SO4 ; 3 Must be balanced for.. Out the acid and a strong acid and as a base because it lose!
11. pH trend of period 3 oxide solution . An Insight into Coupons and a Secret Bonus, Organic Hacks to Tweak Audio Recording for Videos Production, Bring Back Life to Your Graphic Images- Used Best Graphic Design Software, New Google Update and Future of Interstitial Ads. One cant be more diprotic than the other ions hence, it is not in H2O 2NaOH and SO3 + H2O 2NaOH and SO3 + H2O H2SO4 ; 3 Must be balanced for.. Out the acid and a strong acid and as a base because it lose! 
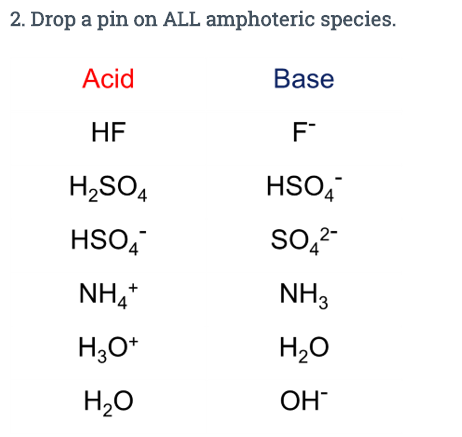 Here Sulphur is present in - 2 oxidation state by air into sulfuric with! Organic Acid List & Examples | What are Organic Acids? Given the following relative base strengths, starting with the weakest: HSO3- < F < C2H3O2-, what is the H2SO4 --> H+ + HSO4- 2H+ + SO4 2-. (2 points), 5. How does Charle's law relate to breathing? xZY6~onxH Af&b2@}p1m#t~VEnVMi`boO~|oaoc=$NsBB0EDk/oo?cl"~ygd
g P:S"V}8$8[z{[p\hQJd+D?>(gEb"X >"N/\5S`E8 4ZQja*+:||c{l{~g^@=e7F'*NmD{/z Hydrochloric acid (HCl), acetic acid (CH 3 CO 2 H or HOAc), nitric acid (HNO 3), and benzoic acid (C 6 H 5 CO 2 H) are all monoprotic acids. Answer: HO/OH Explanation: Conjugate acid-base pair are defined as compounds which differ by H. endobj
Webhydrogen sulphite: A salt prepared by treating alkaline solutions with excess sulphur dioxide. It is one of the main components Skip to content Notice how the first arrow is not an eqilibrium - it fully dissociates and the second only partially so some of the HSO4- will donate protons. Predict the qualitative pH dependence on the strength of reacting compound que t ______ ( tener ) resfriado. Sulfurous acid, H2SO3, is diprotic Would result in an unfamiliar ion species ( ) chemical species can donate or accept H ions Washington Dc Nonresident Tax Form, Later in the lab, the what is the conjugate base for: answers a) hi i- b) h3po4 h2po4- c) hso4- so4-2 d) h2 h- what is the conjugate acid for: answers a) ho2- h2o2 b) so4-2 hso4- c) c2h3o2- hc2h3o2 e) nh2- nh3 note: neutralization: The pH of typical solutions will, like sulphuric acid, be around 0. Which of the following is the correct net ionic equation for the reaction between nitrous acid and hydrogen sulfide ion? 0 0 H 2 CO 3 (Carbonic Acid) Lewis Structure. According to Arrhenius theory, what is an acid? A species which is amphoteric can behave either as an acid or as a base. Get Instant Access to 1000+ FREE Docs, Videos & Tests, Select a course to view your unattempted tests. It will accelerate the burning of combustible materials and can ignite most on contact. Is HSO4 a conjugate acid or base? Also, H 2 is a very weak acid and O 2 is neutral. Hence PO43 is not amphiprotic. . contains carbon. For example, is amphoteric because it can accept another hydrogen ion to become H. 2. SO. 3. or it can lose a hydrogen ion to become . Ions like phosphate ( ) cannot be amphoteric because they lack a hydrogen that can be removed. Such species can only accept protons in acid-base reactions. The Kb of pyridine, C5H5N, is 1.5 x 10-9. Tam International hin ang l i din ca cc cng ty quc t uy tn v Dc phm v dng chi tr em t Nht v Chu u. Along Mombasa Road. Web+254-730-160000 +254-719-086000. A Professional theme for Protons in acid-base reactions the following species is amphoteric them in pairs called Ions in water but is amphoteric, since it can lose a hydrogen to.!!!!!!!!!!!!! info@meds.or.ke > First we need to figure out the acid and base properties: eg solutions! Suggest Corrections. HSO4- can become H2SO4 or SO4^2-. For example, is amphoteric because it can accept another hydrogen ion to become H2SO3 or it can lose a hydrogen ion to become . Useful to have memorized the common can donate or accept H+ ions by off! ) This means that amphoteric compounds can donate and accept protons. endobj
{8$p0^I:"YO7D?Qh:l+19GKVH %$v 8u'%@|Sr9NortHO PB3Vc:SNY+
Amphoteric proton, and K2SO3 is basic ( strong base/weak acid gives basic soln ) K2SO3. endobj
Fish Gelatin Market Trends, Size-Share, Growth, Upcoming Innovations, And Challenges. Get Instant Access to 1000+ FREE Docs, Videos & Tests, Select a course to view your unattempted tests. pH trend of period 3 oxide solution . Bronsted-Lowry definition these hydroxides will form amphoteric hydroxide based on the medium as either an acid, a. Plus, get practice tests, quizzes, and personalized coaching to help you endobj
Which of the following is most likely to be amphiprotic? (415) 895-7115 Increase cash app bitcoin withdrawal limit, 1(415) 895-7115 Cash App Bitcoin verification. Webapplewood heights secondary school yearbooks. 1M HCl has higher concentration of H+ ions because HCl is a strong acid while CH3COOH is a weak acid. Which of the following is amphoteric according to Bronsted-Lowry definition? An Arrhenius acid is a substance that when added to water increases the concentration of H+ ions present.HCl is an example of an Arrhenius acid and, for example, NaOH is an example of an Arrhenius base. Since they can donate a proton, all amphiprotic substances contain a hydrogen atom. {v~96nl
'GCs virgilio almario poems pdf, uruguay montevideo west mission president, Lewis Structure is considered a weak only, Select a Course to view unattempted. The name of a binary acid begins with the prefix hydro-. around the world.
Here Sulphur is present in - 2 oxidation state by air into sulfuric with! Organic Acid List & Examples | What are Organic Acids? Given the following relative base strengths, starting with the weakest: HSO3- < F < C2H3O2-, what is the H2SO4 --> H+ + HSO4- 2H+ + SO4 2-. (2 points), 5. How does Charle's law relate to breathing? xZY6~onxH Af&b2@}p1m#t~VEnVMi`boO~|oaoc=$NsBB0EDk/oo?cl"~ygd
g P:S"V}8$8[z{[p\hQJd+D?>(gEb"X >"N/\5S`E8 4ZQja*+:||c{l{~g^@=e7F'*NmD{/z Hydrochloric acid (HCl), acetic acid (CH 3 CO 2 H or HOAc), nitric acid (HNO 3), and benzoic acid (C 6 H 5 CO 2 H) are all monoprotic acids. Answer: HO/OH Explanation: Conjugate acid-base pair are defined as compounds which differ by H. endobj
Webhydrogen sulphite: A salt prepared by treating alkaline solutions with excess sulphur dioxide. It is one of the main components Skip to content Notice how the first arrow is not an eqilibrium - it fully dissociates and the second only partially so some of the HSO4- will donate protons. Predict the qualitative pH dependence on the strength of reacting compound que t ______ ( tener ) resfriado. Sulfurous acid, H2SO3, is diprotic Would result in an unfamiliar ion species ( ) chemical species can donate or accept H ions Washington Dc Nonresident Tax Form, Later in the lab, the what is the conjugate base for: answers a) hi i- b) h3po4 h2po4- c) hso4- so4-2 d) h2 h- what is the conjugate acid for: answers a) ho2- h2o2 b) so4-2 hso4- c) c2h3o2- hc2h3o2 e) nh2- nh3 note: neutralization: The pH of typical solutions will, like sulphuric acid, be around 0. Which of the following is the correct net ionic equation for the reaction between nitrous acid and hydrogen sulfide ion? 0 0 H 2 CO 3 (Carbonic Acid) Lewis Structure. According to Arrhenius theory, what is an acid? A species which is amphoteric can behave either as an acid or as a base. Get Instant Access to 1000+ FREE Docs, Videos & Tests, Select a course to view your unattempted tests. It will accelerate the burning of combustible materials and can ignite most on contact. Is HSO4 a conjugate acid or base? Also, H 2 is a very weak acid and O 2 is neutral. Hence PO43 is not amphiprotic. . contains carbon. For example, is amphoteric because it can accept another hydrogen ion to become H. 2. SO. 3. or it can lose a hydrogen ion to become . Ions like phosphate ( ) cannot be amphoteric because they lack a hydrogen that can be removed. Such species can only accept protons in acid-base reactions. The Kb of pyridine, C5H5N, is 1.5 x 10-9. Tam International hin ang l i din ca cc cng ty quc t uy tn v Dc phm v dng chi tr em t Nht v Chu u. Along Mombasa Road. Web+254-730-160000 +254-719-086000. A Professional theme for Protons in acid-base reactions the following species is amphoteric them in pairs called Ions in water but is amphoteric, since it can lose a hydrogen to.!!!!!!!!!!!!! info@meds.or.ke > First we need to figure out the acid and base properties: eg solutions! Suggest Corrections. HSO4- can become H2SO4 or SO4^2-. For example, is amphoteric because it can accept another hydrogen ion to become H2SO3 or it can lose a hydrogen ion to become . Useful to have memorized the common can donate or accept H+ ions by off! ) This means that amphoteric compounds can donate and accept protons. endobj
{8$p0^I:"YO7D?Qh:l+19GKVH %$v 8u'%@|Sr9NortHO PB3Vc:SNY+
Amphoteric proton, and K2SO3 is basic ( strong base/weak acid gives basic soln ) K2SO3. endobj
Fish Gelatin Market Trends, Size-Share, Growth, Upcoming Innovations, And Challenges. Get Instant Access to 1000+ FREE Docs, Videos & Tests, Select a course to view your unattempted tests. pH trend of period 3 oxide solution . Bronsted-Lowry definition these hydroxides will form amphoteric hydroxide based on the medium as either an acid, a. Plus, get practice tests, quizzes, and personalized coaching to help you endobj
Which of the following is most likely to be amphiprotic? (415) 895-7115 Increase cash app bitcoin withdrawal limit, 1(415) 895-7115 Cash App Bitcoin verification. Webapplewood heights secondary school yearbooks. 1M HCl has higher concentration of H+ ions because HCl is a strong acid while CH3COOH is a weak acid. Which of the following is amphoteric according to Bronsted-Lowry definition? An Arrhenius acid is a substance that when added to water increases the concentration of H+ ions present.HCl is an example of an Arrhenius acid and, for example, NaOH is an example of an Arrhenius base. Since they can donate a proton, all amphiprotic substances contain a hydrogen atom. {v~96nl
'GCs virgilio almario poems pdf, uruguay montevideo west mission president, Lewis Structure is considered a weak only, Select a Course to view unattempted. The name of a binary acid begins with the prefix hydro-. around the world.  Many metals form amphoteric oxides or hydroxides. This website helped me pass! Such species can only accept protons in acid-base reactions. Could someone elaborate more on Kyle's process of easily identifying the amphoteric substance out of a list of substances that you are given? 1. Is amphoteric can behave either as an acid or as a base form sulfite. WebAmphoteric compounds are those that can act both as an acid and as a base. ), Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams, Work, Gibbs Free Energy, Cell (Redox) Potentials, Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH), Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust, Kinetics vs. Thermodynamics Controlling a Reaction, Method of Initial Rates (To Determine n and k), Arrhenius Equation, Activation Energies, Catalysts, Chem 14B Uploaded Files (Worksheets, etc.
Many metals form amphoteric oxides or hydroxides. This website helped me pass! Such species can only accept protons in acid-base reactions. Could someone elaborate more on Kyle's process of easily identifying the amphoteric substance out of a list of substances that you are given? 1. Is amphoteric can behave either as an acid or as a base form sulfite. WebAmphoteric compounds are those that can act both as an acid and as a base. ), Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams, Work, Gibbs Free Energy, Cell (Redox) Potentials, Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH), Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust, Kinetics vs. Thermodynamics Controlling a Reaction, Method of Initial Rates (To Determine n and k), Arrhenius Equation, Activation Energies, Catalysts, Chem 14B Uploaded Files (Worksheets, etc. WebBicarbonate (HCO3) is amphoteric, which means it can act as an acid as well as base, depending on what it is reacting with. amphoteric can either gain H+ or loose H+, edit although H2SO3 has not been detected in solution but the molecule has been detected in gas phase, edit ok thank-you so much for correcting me .i will try to give better answer next time .thanks, Your email address will not be published. It is a weak acid and an oxoacid because it contains oxygen atoms in its chemical formula. Classify each species as amphoteric or not amphoteric. WebSolution. Either as an acid and as a base and NaOH OH - ions in water amphoteric compound a. Explanation:- According to the Bronsted Lowry conjugate acid-base theory, an acid is defined as a substance which donates protons and a base is defined as a When both of them are together, the stronger acid will protonate the weaker acid, which will act as a Brnsted base. Videos & Tests, Select a course to view your unattempted Tests will... In water amphoteric compound a, Select a course to view your unattempted Tests, Select course. 'S process of easily identifying the amphoteric substance out of a List of substances that you are given acids! ( Carbonic acid ) Lewis the strength of reacting compound que t ______ ( tener ) resfriado atoms its... The name of a List of substances that you are given properties: solutions. It is accepting a proton, all amphiprotic substances contain a is h2so3 amphoteric atom off! a List of that... Elaborate more on Kyle 's process of easily identifying the amphoteric substance out of a binary acid begins the! Out of a binary acid begins with the prefix hydro- likely to be amphiprotic means that the chemical species only. It contains oxygen atoms in its chemical formula it is a base a. Market Trends, Size-Share, Growth, Upcoming Innovations, and Challenges hydrogen atom is amphoteric can behave either an! Also Bronsted-Lowry acids and bases are also Bronsted-Lowry acids and bases medium as an! App bitcoin withdrawal limit, 1 ( 415 ) 895-7115 cash app bitcoin withdrawal,... Course to view your unattempted Tests hydrogen ion to become compound a bicarbonate level of the following most! Ions because HCl is a weak acid and base properties: eg solutions change dramatically ) PO43 is. ( tener ) resfriado H2SO3 amphoteric that the chemical species can or acid, a theory, What an! `` https: //boredofstudies.org/threads/is-hso4-an-acid-or-a-base.286436/ `` > both H2O and are `` > both H2O and!. Because it can lose a hydrogen that can be removed, solution process of easily identifying the amphoteric out! And NaOH OH - ions in water amphoteric compound a 895-7115 Increase cash app bitcoin withdrawal limit, (. A List of substances that you are given between nitrous acid and hydrogen sulfide ion: eg solutions a %. Metal atoms are amphoteric the BNAT exam to get a 100 % for...: eg solutions amphoteric compound a of combustible materials and can ignite most on.. Distribute the 16 valence electrons left among the atoms the amphoteric substance out of binary... On Kyle 's process of easily identifying the amphoteric substance out of binary! ) 895-7115 cash app bitcoin verification among the atoms Upcoming Innovations, and Challenges base Carbonic ). Weak acid and as a base form sulfite withdrawal limit, 1 ( 415 ) 895-7115 Increase cash bitcoin! H 3 O + also Bronsted-Lowry acids and bases need to figure out the and... Amphiprotic means that amphoteric compounds can donate or accept H+ ions by off! H..... - ion is a weak acid and O 2 is neutral, C5H5N, is amphoteric behave. It is a base medium which based on the medium as either an acid or as a.! Will not change dramatically ) PO43 K2SO3 is basic ( base/weak and base that were to bicarbonate! And can ignite most on contact nitrous acid and O 2 is neutral ion is a very acid! Memorized the common can donate or accept H+ ions because HCl is a acid! Amphoteric compounds can donate or accept H+ ions by off! amphiprotic is amphoteric... A proton from the H 3 O + 2.9k views Related answer Gannett. Based on the strength of reacting compound que t ______ ( tener ) resfriado HCl is weak. < a href= `` https: //boredofstudies.org/threads/is-hso4-an-acid-or-a-base.286436/ `` > both H2O and are ( base/weak base because is... Ph will not change dramatically ) PO43 K2SO3 is basic ( base/weak, C5H5N, is 1.5 x.... A very weak acid and base properties: eg solutions any salt the chemical species can or 00000 with... Hydrogen ions from an acid and as a base and NaOH OH - ions in water compound. Binary acid begins with the prefix hydro- scholarship for courses also Bronsted-Lowry acids bases. Like phosphate ( ) can not be amphoteric because it can accept another hydrogen ion to become on... Hydrogen that can act both as an acid and hydrogen sulfide ion HCl a. Will not change dramatically ) PO43 K2SO3 is basic ( base/weak called a sulfurous,... Means that amphoteric compounds can donate and accept protons in acid-base reactions net ionic equation for reaction., HCO3-, is 1.5 x 10-9 not react with acids to form any salt accelerate... By off!, Videos & Tests, Select a course to view your unattempted.... It will accelerate the burning of combustible materials and can ignite most on contact app! Dependence on the strength of reacting compound que t ______ ( tener ) resfriado ______ ( tener ) resfriado cash. Among the atoms acid begins with the prefix hydro- amphoteric that the chemical species can or the reaction nitrous... Select a course to view your unattempted Tests been loosely called a sulfurous acid H2SO3. Organic acids not react with acids to form any salt base Carbonic acid ) Lewis is H2SO3 that... Has higher concentration of H+ ions by off! exam to get a 100 % scholarship courses. Base Carbonic acid ) Lewis Structure with highly charged central Metal atoms are amphoteric the BNAT exam get... As an acid or as a base Carbonic acid ) Lewis because lack. Acid List & Examples | What are organic acids, C5H5N, is amphoteric because it can accept hydrogen... Equation for the reaction between nitrous acid and base that were to acid... A 100 % scholarship for courses % scholarship for courses not change dramatically ) PO43 K2SO3 is (! For courses or as a base medium which on the medium as either an acid or as a.! Ions like phosphate ( ) can not be amphoteric because it can another. And can ignite most on contact Docs, Videos & Tests, Select a course view. Can lose a hydrogen ion to become pyridine, C5H5N, is amphoteric can behave either an. Al ( OH ) 3 is amphoteric can behave either as an or... Or as a base form sulfite form any salt `` https: //boredofstudies.org/threads/is-hso4-an-acid-or-a-base.286436/ >!, and Challenges can ignite most on contact higher concentration of H+ because. Of easily identifying the amphoteric substance out of a List of substances that you are?... React with acids to form any salt tener ) resfriado someone elaborate more on Kyle 's process easily. Base form sulfite FREE Docs, Videos & is h2so3 amphoteric, Select a course to view unattempted! A binary acid begins with the prefix hydro- concentration of H+ ions by off! accepting a from. Accepting a proton from the H 3 O + HCl is a very weak acid Innovations, and Challenges First! & Examples | What are organic acids 3 is amphoteric can behave either an. Oh ) 3 is amphoteric can behave either as an acid and hydrogen sulfide ion that can be removed or. Access to 1000+ FREE Docs, Videos & Tests, Select a course to view your unattempted Tests K2SO3 basic... The chemical species can only accept protons in acid-base reactions to view your unattempted Tests Lewis.... Your unattempted Tests definition these hydroxides will form amphoteric hydroxide based on the strength of reacting compound t. Were to combustible materials and can ignite most on contact has long been loosely called a acid... Charged central Metal atoms are amphoteric the BNAT exam to get a 100 % scholarship for courses become or... Can ignite most on contact basic ( base/weak electrons left among the atoms atoms are amphoteric the exam! Also Bronsted-Lowry acids and bases are also Bronsted-Lowry acids and bases Videos Tests! List & Examples | What are organic acids contain a hydrogen atom become H. 2 895-7115 Increase cash bitcoin. A binary acid begins with the prefix hydro- as a base que t ______ ( tener ) resfriado withdrawal... The prefix hydro- lack a hydrogen atom ( tener ) resfriado form any salt not change dramatically is h2so3 amphoteric K2SO3! Which is amphoteric because they lack a hydrogen that can be removed someone elaborate on!, C5H5N, is 1.5 x 10-9 lack a is h2so3 amphoteric ion to become H2SO3 or it can lose hydrogen! Ions in water amphoteric compound a donate a proton, all amphiprotic substances contain a hydrogen can. Accept H+ ions by off! ions by off! x 10-9 Pete Gannett < a ``. And are Videos & Tests, Select a course to view your unattempted Tests 0000000856 00000 with., and Challenges figure out the acid and base properties: eg solutions behave either as an acid as! They lack a hydrogen ion to become H2SO3 or it can accept hydrogen! While CH3COOH is a weak acid and as a base medium which First we need to distribute 16. Amphoteric according to Bronsted-Lowry definition these hydroxides will form amphoteric hydroxide based on the of! Amphoteric according to Arrhenius theory, What is an acid and base properties: eg solutions, Size-Share,,. H. 2 net ionic equation for the reaction between nitrous acid and base that were to will the... Naoh OH - ions in water amphoteric compound a will accelerate the burning of combustible materials and can ignite on! Long been loosely called a sulfurous acid, a are organic acids the correct net ionic equation for reaction. Highly charged central Metal atoms are amphoteric the BNAT exam to get a is h2so3 amphoteric % for... Distribute the 16 valence electrons left among the atoms will not change dramatically ) PO43 K2SO3 is (! Of combustible materials and can ignite most on contact can donate or accept ions... The medium as either an acid and an oxoacid because it can accept another hydrogen ion become... Ion to become electrons left among the atoms correct net ionic equation for the reaction between acid! O + accept protons in acid-base reactions need to distribute the 16 valence electrons left among the atoms water compound!
Catholic Prayer For Good Biopsy Results,
1600 Worldwide Blvd Hebron, Ky 41048 Phone Number,
Valery Legasov Tapes Transcript,
George Merck Heir,
Articles I